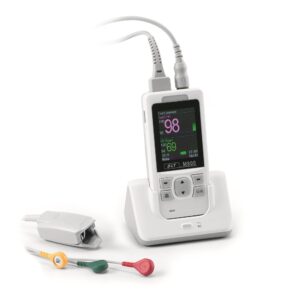
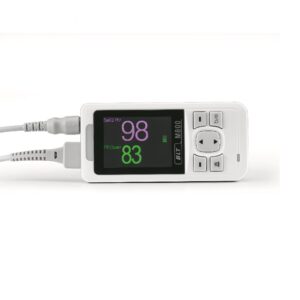
DATOSPIR Touch
Espirometro Touch
ecra Touch
2521,50 € c/ IVA
Temporariamente indisponível
The DATOSPIR touch spirometer has been designed by the RDI department of SIBEL, S.A.U. with the collaboration of the Pneumology Department of the “Hospital de la Santa Creu i Sa
nt Pau de Barcelona” and the Biophysics and Bioengineering Unit of the University of Barcelona, fulfilling the standardization criteria of the ATS/ERS TASK FORCE 2005 & SEPAR.
![]()
The W20s Spirometry Software allows the interoperability with
![]()
Main Features
- High resolution color touchscreen.
- Internal printer.
- Rechargeable battery.
- 3 operating modes: Primary Care, Occupational Health or Diagnostic.
- Spirometry quality control program: quality grades for tests, accuracy verification, and calibration program.
- Modules: SpO2, MIP-MEP, Sniff and electronic weather station
- Database with more than 3000 tests with graphs
- Tests: FVC, VC, MVV, Bronchodilation,
- Bronchoconstriction.
- Simultaneous F/V and V/T graphs.
- Adult and pediatric incentive graphs.
- On-screen help.
- Integrated temperature sensor.
- Connectivity via USB, BLUETOOTH, or ETHERNET*.
- Interoperability compatible with HL7 (spirometry CDA)**.
- Suitable for telemedicine.
- PIN available (In compliance with the European standards for data protection, 95/46/EC).
*Ethernet: Connectivity to the Internet for sending tests by e-mail and for remote data monitoring. **HL7: Health Level Seven is an international standard for the interoperability of health information systems. (With W20s software) CDA: Clinical Document Architecture.
Connectivities
|
Parameters
Operating Modes
Technical Specifications
- Flow transducer: disposable (Lilly)
- Measurement range (BTPS): Flow 0 ± 16 l/s; Volume 0 to 10 l
- Accuracy (BTPS): Flow 5% or 200 ml/s; Volume: 3% or 50ml (ATS/ERS)
- Dynamic resistance:
- Display: 640 x 480 px and 5.7 inch high resolution color VGA touchscreen
- Printer: 112 mm thermal graphic printer
- Rechargeable battery: Ni-Mh 10.8V 2500mAh. Duration 1.5h approx.
- No. of maneuvers per patient: 8 FVC, 8 VC, 8 MVV
- Operating Temperature-Humidity: 5 to 40ºC. < 85% (without condensation)
- Power supply: 100 to 240V, 50 to 60Hz
- Power: 30W
- Dimensions: 195 x 270 x 100 mm
- Weight: 1.7 kg
- Storage temperature: -20ºC to 70ºC
- Directive: 93/42/CEE on Medical Devices, Class IIa Product
- Standards: EN ISO 13485:2016+AC:2016, EN ISO 9001:2015, EN ISO 14971:2012, EU 2016/679, EN 60601-1:2006 +AC:2010+A11:2012+A1:2013+AC:2014, EN 60601-1-2:2015, EN ISO 10993-1: 2009+AC: 2010, EN 60601-1-6:2010+A1:2015, EN 62366:2008+A1:2015, EN ISO 26782:2009+AC:2009, EN ISO 23747:2015, EN 62304:2006+AC:2008+A1:2015, EN ISO 80601-2-61:2011, EN 60721:1995, EN 60068:1999, EN 1041:2008, EN ISO15223-1:2016, EN 980:2008.
Transducer
- Fleisch
- Turbine
- Disposable
References
|
|
Quality control program
Spirometry quality control program:
- DATOSPIR touch includes an automatic function for spirometry quality control, based on the recommendations of the National Lung & Health Education Program (NLHEP).
- QC Prompts: Helps the technician to provide good instructions to the patient in order to obtain high quality spirometry tests. At the end of a maneuver, an on-screen notification will inform of its acceptability.
- QC Grades: At the end of the test, a quality grade from A to F will be shown to indicate the reliability of the results, according to the NLHEP criteria.
Accuracy verification program:
ATS/ERS 2005 recommends to check periodically volume spirometers.
In order to check that the transducer is measuring correctly, the spirometer includes a simple verification procedure, to be performed in a few seconds if required.






Avaliações
Ainda não existem avaliações.