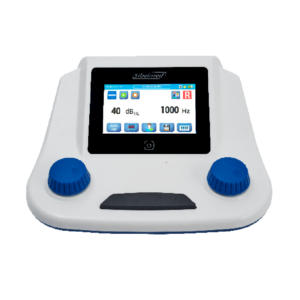
DATOSPIR Aira
Espirometro Computorizado
Conectividade Bluetooth e USB
1291,50 € c/ IVA
Disponível por encomenda
DATOSPIR aira
represents the 8th generation of spirometers developed by SIBELMED’s RDi department, complying with the latest ERS/ATS and SEPAR standards.
Is the only computerized spirometer that combines Bluetooth and USB connectivity and that, at the same time, allows the choice of 3 transducers: DISPOSABLE (Lilly), FLEISCH and TURBINE.
DISCOVER DATOSPIR AIRA

The versatility of the DATOSPIR ![]() makes it the ideal spirometer for occupational health, primary care, pulmonary function units, private practice and platform integration environments.
makes it the ideal spirometer for occupational health, primary care, pulmonary function units, private practice and platform integration environments.
W20S SOFTWARE
DATOSPIR ![]() incorporates as standard the SIBELMED W20s software, to carry out simple intuitive and quality spirometry:
incorporates as standard the SIBELMED W20s software, to carry out simple intuitive and quality spirometry:



ADULT AND PEDIATRIC REFERENCES
GLI/QUANJER · SEPAR 2013 · ERS · KNUDSON · CRAPO · ZAPLETAL · MORRIS · AUSTRIA · GUTIERREZ · CASTRO – PEREIRA 2002 · POLGAR-WEN · GHANKINSON-NHANES III · PEREZ-PADILLA · A.J. CRUZ GOLSHAN · GARCIA RIO · CANDELA · PLATINO · THAI 2000 · CASTRO PEREIRA 2007
TECHNICAL SPECIFICATIONS, TESTS AND PARAMETERS
| Technical specifications | |||
|---|---|---|---|
| Models by transducer | Disposable | ||
| Weight (g) | 124 | ||
| Dimensions (cm) | 15,5 x 10 x 3,5 | ||
| Measurement range (BTPS) Flow Volume |
0 a ±16 l/s 0 a 10 l |
||
| Resistance to: 14 l/s (cmH20 / l/s) |
<1,2 | ||
| Measurement accuracy (BPTS) Volume Flow PEF |
(whichever is greater) 3% o 50 ml 5% o 200 ml/s 10% o 300 ml/s (ATS/ERS 2005) 10% o 170 ml/s (EN ISO 23747:2015) |
||
| Connectivity | Bluetooth 2.0 USB 2.0 and 3.0 |
||
| Batteries | 2 alkaline AAA batteries (standard) 2 rechargeable AAA batteries |
||
Tests and parameters
FVC / Broncodilatación
VC
MVV
Broncoconstricción
More than 40 parameters




Avaliações
Ainda não existem avaliações.